Bureaucratic Failures Left Nigeria’s Leprosy Patients Suffering—Now WHO Rushes to Fix the Mess
The World Health Organization (WHO) will ship leprosy drugs to Nigeria this weekend, ending a year-long delay that left thousands of patients, including children, without treatment and at risk of severe disability.
CABS World News gathered that Nigeria, which records over 1,000 new leprosy cases annually, exhausted its supply of multi-drug therapy (MDT) in early 2024. Bureaucratic holdups and new domestic testing regulations stalled shipments from India, where a key component of the medication is produced.
A WHO spokesperson confirmed to AP that a batch of drugs is set to leave India on March 8, arriving in Nigeria on March 9. The United Nations health agency, which coordinates global leprosy drug distribution, had requested an exemption from Nigeria’s new testing rules. The waiver was granted in January, clearing the way for delivery.
At ERCC Hospital in Nasarawa, west of Abuja, only two leprosy patients remained when AP visited in February. A shortage of drugs forced the hospital to send home 26 others over the past year, increasing the risk of disease transmission.
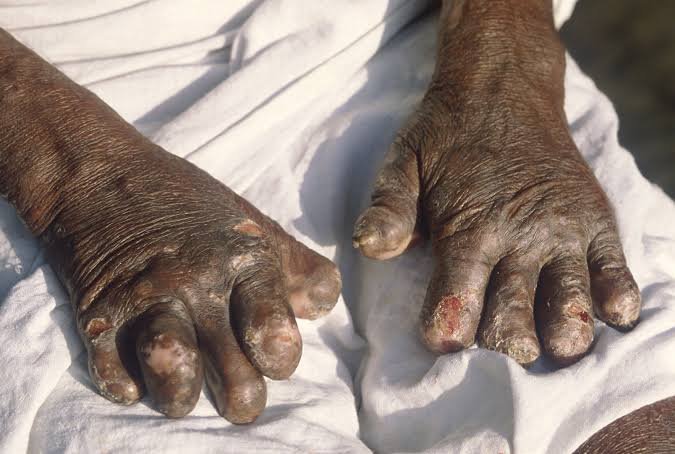
Awwal Musa, a patient whose treatment was interrupted, described her worsening condition. “Before last year, my wounds were healing. Now, they are getting worse. The pain is worse,” she said. Musa’s fingers have curled into claws, and her legs are covered in infected sores.
Doctors say they have been working to prevent permanent disabilities among patients. “If they lose their fingers, who will give them new ones? If they lose their sight, who will restore it?” said Kuzeh Thomas, a hospital director.
Nigeria is one of 12 countries that report between 1,000 and 10,000 new cases of leprosy annually. MDT, donated by Swiss pharmaceutical company Novartis, is distributed globally through a WHO program. Each country must request its yearly supply, but health sources say Nigeria’s request was delayed.
Nigeria’s National Agency for Food and Drug Administration and Control (NAFDAC) required additional testing for doses produced by Sandoz, a Novartis spin-off, because they were manufactured in India. The drugs arrived in Nigeria in November 2024 and were finally approved for use in December.
Novartis said it remains committed to eradicating leprosy but did not comment further. Sandoz did not respond to requests for comment.
Beatriz Miranda-Galarza, the U.N. special rapporteur on leprosy discrimination, warned that while the global drug distribution system appears well-structured on paper, “it faces significant structural and political challenges in practice.”
“This is the first time we are seeing such a painful situation,” said Sunday Udoh, head of Leprosy Mission Nigeria. “Leprosy patients, among the poorest of the poor, have been left without life-saving medication.”